The unlikely, inevitable founding of PopVax, Part I: Three meetings and six million funerals
Broadly-protective mRNA vaccines to save millions of lives
In 1992, an electronics engineer and failed battery entrepreneur met the legendary Kallam Anji Reddy, the founder and namesake of Dr. Reddy’s Labs, then as now one India’s largest pharmaceutical companies, to pitch him on an idea for a new vaccine venture.
In 2000, the Lithuanian-born Muslim-Jewish chairman of a storied Bombay enterprise attended a meeting of the European Commission, where he gave a speech that shook up the proceedings and changed the course of medical history.
In 2021, the son of two vaccine-skeptical homoeopaths travelled to Hyderabad for the first time to meet the former CEO of a pioneering Indian vaccine company at a coffee shop.
Today, in August 2023, I, Soham Sankaran, a lapsed computer science researcher, run PopVax, an Indian biotechnology startup based in Hyderabad focused on broadly-protective mRNA vaccines. I started this company two years ago with no knowledge of biology since high school beyond trying to read the occasional interesting academic paper I came across, and so little memory of organic chemistry that I’d entirely forgotten that the lines in structural formulae represent carbon-carbon bonds. Nevertheless, PopVax’s 50-person operation has quickly become one of the leading centres of vaccine research and development in the country, working at the frontier of translational biology.
That may sound pretty unbelievable, but those three meetings in 1992, 2000, and 2021, coupled with six million preventable deaths that happened in between, make the unlikely tale I’m about to tell seem, in retrospect, inevitable.
Before we begin in earnest, I want to tell you a little bit more about our work at PopVax. Since January 2022, PopVax has been building a new mRNA platform for low-cost broadly-protective vaccines via computationally-driven antigen design, starting with an open-source booster vaccine candidate that is intended to protect against the entire betacoronavirus genus, including current and future strains of SARS-CoV-2, the virus which causes COVID-19, SARS-CoV-1, which caused the original 2002-2004 severe acute respiratory syndrome outbreak, and MERS-CoV, which causes a deadly disease that has killed one-third of all known cases. Our work is funded via research agreements with the Bill & Melinda Gates Foundation and Ethereum co-founder Vitalik Buterin’s biosecurity fund Balvi, and we were incubated at the CSIR-Centre for Cellular and Molecular Biology’s Atal Incubation Centre.
Our small team of about 50 wet lab scientists, computational biologists, and vaccine manufacturing experts has made an immense amount of progress in a short period of time with a relatively small amount of funding:
We have set up our own mRNA-LNP platform, including our own library of novel ionizable lipids and formulations for nucleic acid delivery, and demonstrated excellent delivery and protein translation in vitro and in vivo.
We have designed, and are in the midst of optimizing, a novel antigen using a mix of cutting-edge computational and experimental techniques to elicit a broadly-neutralizing antibody response to the betacoronavirus genus of the coronavirus sub-family of viruses, and we expect this response to be robust to new variants of SARS-CoV-2 (COVID-19).
We have immunogenicity data showing that the v3 version of our mRNA-encoded antigen is generating strong neutralizing antibody responses to the SARS-CoV-2 Spike protein in animals – sera from BALB/c mice immunized with PopVax’s lead previous generation mRNA construct (v3) neutralizes multiple variants of SARS-CoV-2 pseudovirus orders of magnitude more potently – wild-type (10x more potently), Lambda (45x more potently), and Gamma (120x more potently) – than sera from mice immunized with the same dosage of an mRNA sequence from one of the US-approved COVID-19 vaccines (identical LNP formulations were used for both groups):
We have designed a manufacturing process for our clinical trial lots, and have now built out a GMP-ready pilot-scale facility to house this process in Hyderabad’s Uppal industrial area, which we will soon be ready to run validation batches.
We have received positive responses from the US FDA (via the pre-IND process) on the science behind the candidate, our pre-clinical study plan, and our manufacturing process, laying the groundwork for an IND filing for our Phase I First-in-Human (FIH) trial in the United States.
All in all, we are hoping to be ready to conduct those Phase I trials early next year in Baltimore, Maryland, which, if all goes well, gives us a chance to be the first commercially-available broadly-neutralizing pan-variant (of SARS-CoV-2)- and pan-betacoronavirus vaccine across the world.
We believe our manufacturing process and supply chain will scale smoothly enough to allow us to eventually deliver cutting-edge vaccines for <$1/dose in India and across the developing world, and we believe we can be ready to scale up on the same timeline as the completion of our clinical trials for this candidate.
Why bother developing a new COVID-19 vaccine this late in the game? Well, even leaving aside that there has been a recent uptick in COVID-19 cases across the world, and hospitalizations in the United States in particular, there’s a large and growing body of evidence suggesting that even seemingly mild COVID-19 infections, which people are getting repeatedly across the world, have long-lasting symptoms in an appreciable fraction of people, and a world in which we all get this disease multiple times per year is likely to be a significantly less healthy one in the long run, not least because of a highly elevated risk of heart attacks and other cardiac incidents for 25-50 year old adults in particular after an infection. Even if that scenario seems ‘mild’, remember that all we need is another deadly new variant for us to be plunged back into a 2020-esque pandemic reality all over again, and there is recent evidence (as of mid-August 2023) that BA.2.86, a highly-mutated new variant with a potentially dangerous set of mutations, has started to spread. The sooner we can create an immune response that is robust to a wide enough range of mutations that the virus cannot find its way around that barrier, the better. PopVax’s booster candidate aims to do just that. COVID-19 aside, the betacoronavirus genus has of late given us SARS-CoV-1, MERS-CoV, and now SARS-CoV-2 – if we succeed in our aim of achieving pan-betacoronavirus protection, we may be able to prevent the emergence of a new pandemic-potential pathogen from that genus before it has a chance to spread.
We intend to bring the same technologies and strategies to novel broadly-protective vaccines for a variety of other diseases and disease families, including but not limited to influenza (in particular the dangerous H5N1 strain that may now for the first time be exhibiting human-to-human transmission), tuberculosis (existing vaccines do not effectively prevent adult pulmonary TB), Nipah & Hendra (emerging pandemic-potential pathogens which are of particular interest in South Asia and Oceania), and herpesviruses including HSV, CMV, and EBV (the last of which recent evidence strongly suggests is a necessary precondition for multiple sclerosis). All of these have the potential to be first-in-the-world vaccines, and they could cumulatively save millions of lives, both now and in the future, while protecting millions more from long term disability.
We’re building a world-class, R&D-driven mRNA biopharma company right here in India, which is bursting with talent that the incumbents are both misusing and underfunding. We intend to leverage our platform and this latent talent base to produce viable rationally-designed vaccine and therapeutic candidates to prevent or treat life-threatening diseases at a pace heretofore unseen in the history of the pharmaceutical industry, then distribute the ones which work at massive scale and low cost across the world, with a particular emphasis on ensuring timely and affordable access in lower-income countries. If this piques your interest, we’re hiring aggressively across antigen & protein design, machine learning, nucleic acid delivery, bioassays, immunology, software engineering, communications & scientific writing, and regulatory affairs (especially people with extensive experience getting new vaccines or biologics through the US FDA approval process) – you can take a look at open positions over at jobs.popvax.com. If you believe you could contribute to the company in some way that’s not captured in those job descriptions, you’re welcome to email a cover letter and resume to jobs@popvax.com.
The Bittersweet Cocktail
Let’s start with the bodies, as all good stories do. The six million departed souls I refer to were not casualties of this global pandemic, the COVID-19 pandemic that has upended our lives and societies over the last three years that we are just now beginning to emerge from, but, rather, the previous one – HIV/AIDS, which has taken 40 million lives, give or take, placing it in the same league as the Spanish flu and the first emergence of the bubonic plague.
Starting in the mid-80s, the number of deaths from HIV/AIDS in the United States started to skyrocket, from ~8000 in 1985 to a peak of ~50,000 around 1995. Unlike many other infectious diseases, which disproportionately affect the elderly, AIDS was particularly cruel in its choice of victims – deaths were heavily skewed toward people in the prime of their lives, making it the leading cause of death among Americans between the ages of 25 and 44 by 1994.
Then, even more suddenly, mortality from AIDS fell off a cliff, with deaths falling from ~50k/year to under 20k/year, more than 50%, in just over a single year. The vast majority of this public health miracle is down to the introduction of combination antiretroviral therapy (ART), commonly referred to as the three-drug ‘AIDS cocktail’ approach, usually consisting of two nucleoside reverse-transcriptase inhibitors and one of a different class of HIV-inhibiting drug. It took HIV/AIDS from a near-certain 10-year death-warrant to something survivable for many, many decades, with a life expectancy not too far off from that of an uninfected individual in most cases.
The development of this treatment regimen, chronicled engagingly in Stephen Fried’s deliciously titled 1997 Washington Post feature Cocktail Hour, hinged on an insight, simultaneously but separately demonstrated in 1995 by the famed AIDS researchers David Ho (of the nonprofit Aaron Diamond AIDS Research Center) and George Shaw (of the University of Alabama), that from the moment of infection, HIV is locked in a constant, evenly-matched struggle with the human body’s immune system – the virus infecting cells to make more and more copies of itself to infect further cells, and the immune system finding and extinguishing those infections and copies, such that in any given two day span half the virions that were present in the blood plasma and half the infected cells that were producing copies of the virus at the beginning of that period have already been destroyed. In order to even just maintain its presence in the body, to ‘survive’, the virus needs to replicate at an utterly incredible rate – “just cranking”, in Ho’s memorable words, “just churning out tens of billions of particles a day, every day”. Initially, this battle reaches a sort of stalemate – the immune system producing the T cells needed to extinguish the virus and the virus depleting the T cells at a similar rate – but eventually, the body’s ability to produce the immune T cells it needs to fight the infection reaches a breaking point, and the virus’ replication is no longer counterbalanced, resulting in the patient progressing to full-blown AIDS, an assured death sentence.
This meant that a sustained attack on viral replication after infection but prior to the loss of this battle between the immune system and HIV – “early and hard”, as suggested in the title of Ho’s seminal 1995 op-ed in the New England Journal of Medicine – might be able to hold AIDS, and thus an early death, at bay. Nucleoside reverse-transcriptase inhibitors such as AZT, initially synthesized by the prolific chemist Jerome Horwitz at the Michigan Cancer Foundation as anti-cancer drugs in the 1960s, are one such means of hindering viral replication, and had by the late 1980s become the frontline treatment for HIV. This class of drugs interferes with the process of turning viral RNA into DNA that can be used to hijack a cell, which they accomplish by being phosphorylated into structures that look very much like nucleotides, the DNA building blocks, to HIV’s reverse-transcriptase enzyme, even though they in are in fact entirely non-functional and halt the chain of viral DNA synthesis whenever they are accidentally integrated into a sequence.
Despite the initial effectiveness of these drugs, the virus would develop resistance to these drugs within the patient’s body in just a few short months. This comes down to the fact that HIV mutates frequently via random reverse-transcription errors – it lacks a proofreading mechanism to check whether the DNA it has copied from its viral RNA is correct, and in doing so produces a diverse set of variants within the body as it replicates. As Ho, Shaw, and company figured out, the virus is replicating a massive amount all the time, with new copies replacing half of the old every two days, so it makes sense that when placed under selection pressure by a single type of drug that hinders replication, it nearly inevitably mutates around it by randomly happening upon a single transcription error at the exact point in its genome that allows it to negate the drug’s effects.
But when simultaneously attacked by, say, three drugs using sufficiently different mechanisms to prevent its replication, “forc[ing] the virus into a corner”, as Ho called it during an interview with PBS, the emergence of one mis-transcribed DNA sequence containing all three mutations (at the very least) at exactly the right places needed to circumvent the barriers posed by those three drugs at the same time is “exceedingly difficult or statistically improbable”.
In the early 1990s, the Canadian pulmonary specialist turned AIDS researcher Julio Montaner had come to the idea of combination therapy via a more practical route – it was a riff on his successful method of treating tricky pneumonia cases, in particular those exacerbated by HIV/AID, with a combination of steroids and antibiotics, itself inspired by his father’s experiences treating stubborn cases of tuberculosis in their native Argentina. He’d tried treating patients with multiple nucleoside reverse-transcriptase inhibitors at the same time, which was better, but still didn’t stop the disease from eventually adapting, in particular because similar mutations could facilitate escape from multiple instances of this class of drug at the same time.
To make combination therapy work long-term, they needed types of drugs that were different enough to ensure that mutation around them at the same time as nucleoside reverse-transcriptase inhibitors was actually as improbable as Ho was hoping. In very short order, they got two – protease inhibitors and non-nucleoside reverse-transcriptase inhibitors, the pioneering research for both of which came not from academia, as would be typical for such advances, and was the case for nucleoside reverse-transcriptase inhibitors, but directly from the labs of private pharmaceutical companies.
Protease inhibitors block the action of the enzyme HIV uses to cut to size the proteins it forces cells to produce from which it assembles copies of itself. In a remarkable triumph of the capitalist model, three companies – Roche, Merck, and Abbott – separately poured staggering amounts of cash and manpower into protease inhibitor R&D from 1986-1996, all of them using a then-untested rational design strategy, where instead of either testing natural molecules for their effect against a given target or performing brute-force combinatorial synthesis and screening, drugs were designed specifically to dock with a particular location on the three-dimensional structure of the target, which was then newly possible to determine precisely using a combination of what were at the time the latest electron microscopy techniques and computational tools.
Despite many setbacks, including the tragic death of Irving Sigal, the Merck chemist who led the team that ‘solved’ that 3D structure, in the Lockerbie airplane bombing at the age of only 35, by 1995 all three companies had succeeded, and trials of all three HIV protease inhibitors as part of combination therapy with nucleoside reverse-transcriptase inhibitors had begun.
Meanwhile, the pharmaceutical firm Boehringer-Ingelheim lost the race to develop a protease inhibitor, but a 1988 side project targeting the reverse-transcriptase enzyme using a combination of large-scale screening and structure-activity relationship (SAR) driven rational analogue development bore fruit, resulting in nevirapine (NVP), the first HIV-1 non-nucleoside reverse transcriptase inhibitor. This class of drugs disables the reverse-transcriptase enzyme in a different way from nucleoside reverse-transcriptase inhibitors, binding to a hydrophobic pocket away from the active site rather than pretending to be and competing with real nucleotides. By 1994, Julio Montaner managed to get his hands on NVP to use as part of a combination therapy trial along with two nucleoside reverse-transcriptase inhibitors.
By early 1996, it was obvious from multiple studies that 3-drug cocktails of two nucleoside reverse-transcriptase inhibitors and either a protease inhibitor or a non-nucleoside reverse-transcriptase inhibitor could suppress replication enough to render the HIV virus almost undetectable in many patients. In quick succession, Roche, Abbott, and Merck all had their protease inhibitors approved – Roche’s saquinavir was approved in December 1995, and Abbott’s ritonavir was approved in March 1996 a mere 72 days after the company applied to the FDA, setting a record for the fastest ever approval, a record that was shattered just weeks later by the approval of the Merck’s indinavir 42 days after the application. This is nearly as fast as the Pfizer-BioNTech mRNA vaccine was granted Emergency Use Authorization in December 2020 (about 3 weeks after application to the FDA). By June 1996, Boehringer-Ingelheim had been granted approval for nevirapine, and the ART era had begun in earnest.
The FDA doesn’t usually move this fast – the speed of these medications getting into general circulation rivals the EUAs granted to the first COVID-19 vaccines in the US in December 2020, which was only palatable to regulators because of the global pandemic situation. What motivated them to help get these drugs to patients at lightning speed? Just as the AIDS cocktail depends on the simultaneous action of three separate drugs, the story I’ve told was the result of the ceaseless work of three different groups of people. Two of those groups we’ve already met – the academic researchers who pioneered the first nucleoside reverse-transcriptase inhibitors as well as the core ideas behind the regimen, and the industrial scientists behind the development of the protease inhibitors and non-nucleoside reverse-transcriptase inhibitors that provided the distinct mechanism of action needed to keep HIV in check. The third group was the incredibly energetic community of patient-activists – individuals living with HIV/AIDS who formed organisations like the AIDS Coalition to Unleash Power, better known as ACT UP, which started in 1987, and the Treatment Action Group (TAG), which splintered off ACT UP in 1992 – who refused to just lie down and die while the government and the FDA moved too slowly to fund and advance through the pipeline treatments that might have the ability to save their lives. One of the more prominent ways they protested regulatory paralysis was, in an intentional act of dark irony, to lie down and play dead in front of government buildings, including the 1988 protest in which they “Seize[d] Control of the FDA”, occupying the compound of the agency’s then-headquarters in Rockville, Maryland.
The ACT UP folks combined this eye-catching style with genuine scientific understanding – its Treatment and Data Committee, informally led by Mark Harrington, constituted a Science Club that kept abreast of the latest developments in HIV/AIDS research, eventually interfacing directly with scientists, regulators, and pharmaceutical companies to help accelerate the progression of lifesaving treatments into the clinic. Harrington and his fellow Science Club members weren’t scientists by training, but their voracious consumption of the scientific literature, deep insight into patient recruitment, and innovative ideas around clinical trial strategy won them the deep respect of their interlocutors, and gave them an opening to advance their agenda of “getting drugs into bodies” as fast as possible. Indeed, the omnipresent Anthony Fauci, who in the late 80s was just a few years into his tenure as the Director of the National Institute of Allergy and Infectious Diseases – he stepped down in December 2022, 38 years after he first got the job – made a serious effort to hire Harrington as a researcher, and was said to be “dazzled by his brilliance”.
These genius of executing these two strategies in tandem was that by the time public pressure forced the FDA to crack open the door to streamlining the approval process, the credibility the scientific side of the activist groups had gained allowed them to swiftly put their foot in the opening and have a real say in reforming what was broken. These discussions led, in 1989, to the creation of the “Parallel Track” program, which allowed for experimental drugs which had passed safety but not yet efficacy studies to be used by patients outside of clinical trials, in 1992, and the “Accelerated Approval” pathway, which allows for the conditional approval of drugs which meet key proxy markers for efficacy in early-stage clinical trials even before large-scale trial data becomes available, setting the stage for the just-emerging protease inhibitors and non-nucleoside reverse-transcriptase inhibitors to speed their way into wide-scale deployment between 1995 and 1996.
You already know the rest – deaths from HIV/AIDS fell by 50% in that year alone. The actions of all three groups – the companies, the researchers, and the activists – formed a sweet symphony of symbiotic scientific and societal strides toward saving the lives of the sick and suffering, culminating in an incredible shared victory. The graph bears looking at again – there are few cleaner representations of the impact of anything on human health.
Tens of thousands of additional lives were saved in the United States – given, in many cases, decades of additional time on this earth – for every year the introduction of these drugs into the clinic was accelerated. Wait two more years, and maybe a hundred thousand additional people die – mostly people between the ages of 15 and 50, teenagers, young adults, middle-aged adults, people in their prime.
Complete vindication, then, for the American system? Companies investing in R&D, academic researchers figuring out how to combine the drugs to make them more than the sum of their parts, patient groups pressuring for faster approvals and greater access, and even regulators creating new pathways for clinical use on the fly – everyone doing their part, checking and balancing each other, the whole puzzle fitting together, and the right thing actually happening? Incredible! Let’s export this model globally!
Ah. ‘Globally’... therein lies the rub.
Here’s another graph covering the same time period, but for a different region – Africa (as defined in the WHO’s regional classification). By 1995, there were 13.7 million HIV+ individuals in Africa – that’s out of a total of 17.6 million globally – and the disease had already become one of the leading causes of death in the region.
Notice anything different about this? Just as in America, deaths rose along with prevalence from 1990 to 1995, but while the American version peaks in 1995-96 and falls off a cliff, the African death rate doesn’t peak till 2005 – about a decade later – and took until 2015, another decade, to decline by 50% from peak, which was achieved in America in a single year. The HIV-survivable future was already here in 1995, but it was very unevenly distributed.
Why? Well, one might blame it all on that old villain of healthcare nightmares – cost. Take, for example, the three-drug combination of stavudine (d4T) + lamivudine (3TC) + nevirapine (NVP). From the introduction of the three-drug ARV regimen in 1995 until October 2000, their combined price never fell below $10,000 per patient per year anywhere in the world, which was completely and utterly unaffordable in developing countries – South Africa’s per capita income at that time was only $3000, and that was by far the highest in Africa at the time. Where did that cost come from? Patents, and their attendant exclusivity. In this case, the patent to each of these medicines was owned by a separate pharmaceutical company – Bristol-Myers Squibb (stavudine), Glaxo Wellcome/GlaxoSmithKline (lamivudine), and Boehringer Ingelheim (nevirapine) – and they were making blockbuster profits – tens of billions of dollars – selling them in the United States and Europe. Despite pressure from developing countries, public health and AIDS activists, and even international bodies like the UN, the pharma companies were unwilling to drop their prices, even in Africa, nor were they willing to license their patents or waive their intellectual property rights to allow other pharmaceutical companies to manufacture and sell these drugs at affordable prices in lower-income countries.
The general pharmaceutical company argument for patents is that without them, there would be no way to price novel medicines high enough to recoup the cost of R&D, and thus there would be no incentive to do the R&D in the first place, since it would be loss-making even if the medicine succeeds, which means that the medicine would never have been developed. This neat argument ignores, of course, that the majority of the R&D funding that eventually leads to new medicines comes from governments and non-profit organizations, and a huge chunk of truly novel work is done not within companies, but at non-profit universities and research institutions. Of course, there are drugs, such as the first set of HIV protease inhibitors, which do come from private R&D fuelled by private expenditure, but it’s certainly not the case that the patent system always or often fairly rewards individuals and organizations for their work and investment – often, those who profit from a patent aren’t the ones who put the effort and resources behind it at all. Consider, for example, nucleoside reverse-transcriptase inhibitors. Remember Jerome Horwitz, the guy who first synthesised AZT? Incredibly, three of the earliest nucleoside reverse-transcriptase inhibitors to be approved – Zidovudine/AZT (the first), Zalcitabine/ddC (the third), and Stavudine/d4T (one of the most widely used), were all first designed and synthesized by Horwitz and his team as anti-cancer drugs in the 1960s, to the point where for a time a leading strategy for the (re)discovery of this class of drugs was to look through Horwitz’s back catalogue and test them against HIV – this is precisely what William Prusoff and Tai-shun Lin did at Yale that led to the repurposing of d4T as an anti-HIV drug. A remarkable documentary by Vincent Detours and Dominique Henry, I am alive today (transcript), tracked down Horwitz and others to piece together the story of how these drugs came to be used as antiretrovirals. Horwitz, who died in 2012, unquestionably helped save tens of millions of lives, but he never patented these drugs, and consequently never made any money off them – it was the scientists and pharmaceutical companies who tested their effect against HIV that received patents for that application, and profited in both money and attention lavished upon them for their application of his seminal work. Indeed, Burroughs Wellcome (later absorbed into Glaxo Wellcome, and later still into what is now GlaxoSmithKline), which made billions off AZT, made a show of ‘thanking’ Horwitz by donating a mere $100,000 to his university with the intent to establish a chair in his name, but didn’t donate any further even when it turned out this wasn’t enough to establish an endowed professorship.
All of that aside, even the most favourable case for patent enforcement depends on the idea that without them, the company that holds the patent would make less money because of competition from other manufacturers. In the case of HIV/AIDS medicines in Africa, with pharmaceutical companies unwilling to lower their prices, there was effectively no market for the drugs – no one could afford them, so the pharma companies were making basically nothing in that market anyway. The removal of their patent protections and the arrival of competing manufacturers in those markets would not have reduced pharma company earnings at all! This placed pharmaceutical companies in the ludicrous position of defending their patents and prices – and thus the continued death of millions of African AIDS victims – on the basis of lost revenue that was entirely fictitious. The real fear, of course, was of the US Congress or the European Parliament, now facing a public aware of the real (low) price of medicine production, tiring of being forced to bear ridiculous per-patient costs, and forcing negotiation to far slimmer margins. In essence, poor Africans had to die so that GSK could keep overcharging rich Americans.
Realizing, one imagines, the utter moral emptiness of that argument, for years pharmaceutical companies tried to blame this indefensible state of affairs on anything other than intellectual property. One repeated tactic was to claim that it was not possible to produce these drugs sustainably and safely at costs considerably lower than they were selling them at. Another was to make risible claims that poorer countries couldn’t properly make use of the HIV antiviral cocktail even if patents weren’t in the way, as in this blood-boiling excerpt from a 1999 article in the San Francisco Chronicle:
The drug manufacturers insist that there are no simple solutions to the international AIDS crisis. The triple-combination drugs that are saving lives in the United States and Europe require sophisticated laboratory tests to monitor the type of drug and dosage. Most poor countries do not even have the money to buy the simplest AIDS diagnostic tests.
Dr. Richard Laing, of the Boston University School of Public Health, said that bypassing patents won't begin to solve the drug access problem in the poorest countries. "The vast majority of drugs on the World Health Organization essential-drug list are off-patent. Despite that, access remains poor."
Laing said that if compulsory licensing is used by developing countries, it will make more sense to apply it to production of the most cost-effective drugs -- those that can cure rather than just control disease. For example, the anti-fungal drug fluconazole can cure a variety of diseases within a short time frame, whereas AIDS antiviral drugs work best only in three-drug combinations, have serious side effects and must be taken over a lifetime.
Conveniently unmentioned by Laing, of course, is that there weren’t and still aren’t any drugs that can fully cure HIV, so his stance was the equivalent of leaving tens of millions of HIV/AIDS victims in Africa, and further tens of millions in Asia and South America, bereft of treatment beyond what meagre donations were directed their way by Western governments and pharmaceutical companies. If diagnostic test prices were indeed the primary problem, one might consider finding a way to make them cheaper – this, of course, was not something the pharma companies wanted to contemplate.
Unfortunately, these hollow and condescending talking points about poor countries’ inability to use ARVs were echoed by US government officials at the time. In a hearing of the House Committee on International Relations excerpted in the excellent HIV/AIDS documentary Fire in the Blood, Andrew Natsios, Administrator of USAID, said that it would be difficult to properly use these medicines in Africa because
People do not know what watches and clocks are. They do not use western means for telling time. They use the sun.
Despite pharma company protestations and diversionary tactics, it was always about inflated and opaque pricing enabled by patents, an obvious fact which the pharma companies validated when, after South Africa passed a 1998 law that would allow the government to buy these and other lifesaving medicines – brand name, mind you, only from authorized manufacturing sites operated or licensed by the originators themselves – at the lowest available global price and import them into the country, as well as to issue compulsory licences to local manufacturers during public health emergencies, global pharma companies led by Bristol-Myers Squibb, SmithKline Beecham, Glaxo Wellcome, Bayer, and Roche decided to sue the South African government, calling the law unconstitutional. In what can only be described as a stunning lack of self-awareness, the drug companies named as the first respondent and principal defendant of this lawsuit a man with some passing familiarity with facing morally-bankrupt charges from white oppressors – one Nelson Mandela, then President of South Africa.
Then, a miracle!

In October 2000, the ‘originator’ drug companies behind these three medications collectively made price reductions resulting in a cost of just under $1000 per patient per year in Africa, a reduction of 90% of the cost. What happened that changed their minds all of a sudden? The story of this incredible act of seeming charity is that these companies had no choice.
The Pirates of Bombay
“Friends, I represent the third world”
Seated between representatives of Glaxo and Merck, the speaker was an interloper at the September 2000 meeting of the European Commission.
“I represent the needs and aspirations of the third world”
This was not a new position for him. The inheritor of Cipla, an Indian pharmaceutical company founded on Gandhian principles of national self-sufficiency, Yusuf K. Hamied was born to a Lithuanian Jewish mother and an Indian Muslim father in Vilnius, raised in a house by the sea in my hometown of Bombay, and lives an itinerant life between that city, Mauritius, London, and Marbella.
“I represent the capabilities of the third world”
And he was here, on the advice of the activist Jamie Love, then as now one of the most prominent voices fighting for equitable access to medicine and reform of the global patent system, Bill Haddad, a chameleonic investigative journalist and Washington insider, and Médecins Sans Frontières (MSF), better known as Doctors Without Borders, to call the pharmaceutical industry’s bluff. Assisted by the pioneering synthetic chemist A.V. Rama Rao, Cipla was able to manufacture the HIV cocktail at an affordable price, and Hamied was offering to sell it at $800 per patient per year, down from the $10k+ charged by the ‘originators’.
“And above all, I represent an opportunity. We all have a responsibility to alleviate the suffering of millions of our fellow men who are afflicted with HIV & AIDS.”
Suddenly, the calculus was clear – save lives or continue to sacrifice HIV/AIDS patients at the altar of patents and profit.
“We call upon the participants of this conference to do what their conscience dictates”
Motivated by the massive global backlash against their self-immolatory decision to sue the South African government, 5 pharmaceutical companies making components of various versions of the ARV cocktail had already announced, in May of 2000, that they would partner with the United Nations and the World Bank to make their medicines available at a discount to some countries, but in the months since then negotiations on the exact details of those discounts had slowed to a snail’s pace, with the companies forcing each country to negotiate separately, refusing to allow the prices arrived at to be publicly revealed, and only making a very small quantity of discounted medicines available as part of the program.
Now cornered by Hamied, the companies started to allow those talks to progress towards completion, resulting in an agreement with Senegal in October to discount a limited supply of the medicines to just over ~$900 per patient per year (this is October 2000 ‘originator’ price drop in the graph).
But Hamied wasn’t done yet. Frustrated that no government had reached out to him to acquire the HIV cocktail at the 800 price, he hatched an even more audacious plan with Love, Haddad, and MSF. In February 2001, he announced that Cipla would provide the cocktail to MSF at $350 per patient per year – less than $1 a day to save a human life.
This move, which was reported on the front page of the New York Times, made Hamied and Cipla darlings of the public health world and the scourge of global pharma companies. The head of GlaxoSmithKline, the irascible Frenchman Jean-Pierre Garnier, called them “pirates” and decried that they “never done a day of research in their lives”, an accusation that carries more than a tinge of irony given that one of Glaxo’s key HIV antiretrovirals, by way of the company’s merger with Burroughs Wellcome, was AZT, first synthesized by the deeply undercompensated Jerome Horwitz – it was not a compound they designed, and the pioneering clinical trial that sensationally proved its efficacy was the brainchild of Dr. Samuel Broder at the National Cancer Institute, a government research lab which also conducted the actual study. In fact, the US government intended to apply for the patent on the use of AZT against HIV, but they were controversially beaten to it by Burroughs Wellcome, a situation with striking similarities to the recent dispute between Moderna and their collaborators at NIH on the intellectual property behind their COVID-19 vaccine mRNA sequence design.
Whatever the pharma companies called Hameid, it was too late to stop him – at the (literally) headline-grabbing price-point of $1 per day, the idea of not acting to save these lives became too conscience-denying to bear for the global public. There were demonstrations on the streets in South Africa, in Britain, and in the United States, where ACT UP and assorted allies took over GlaxoSmithKline’s investor relations office in New York while chanting “GlaxoSmithKline! GlobalSerialKiller”.
In March 2001, the first crack in the pharmaceutical industry’s united front became visible. Bristol-Myers Squibb, the owners of the patent on d4T granted based on the work of Yale scientists excavating Jerome Horwitz’s back catalogue, made the decision to stop enforcing their exclusivity rights on the medicine in Africa, allowing low-cost generics such as Cipla’s to enter the market without hindrance.
''This is not about profits and patents,'' said John L. McGoldrick, executive vice president at Bristol-Myers. ''It's about poverty and a devastating disease. We seek no profits on AIDS drugs in Africa, and we will not let our patents be an obstacle.''
At around the same time, Merck began offering its protease inhibitor in Africa for less than $1/day. By April 2001, the pharmaceutical companies saw the writing on the wall and dropped their lawsuit against the South African government.
Throughout all of this, GlaxoSmithKline continued to claim that patents were “not the issue”, with access to medicines, and that their secretly negotiated government-specific limited-supply price reductions were as good as it could possibly get. While the other pharmaceutical companies either promised not to enforce their patents in Africa or worked out deals with Cipla and other low-cost manufacturers, like the Hyderabad-based firms Aurobindo and Hetero, that poured into the market as the floodgates opened, GSK and Boehringer Ingelheim sat apart and bided their time. It took a canny legal stratagem from a homegrown South African activist group, Zackie Achmat’s Treatment Action Campaign, to get them to cave – in 2002, TAC filed an antitrust complaint with South Africa’s Competition Commission claiming the two pharma majors were abusing their dominant position in the ARV market to charge customers extortionate prices while refusing to license their patents. After conducting an investigation, in 2003 the Commission largely accepted that argument and referred the case to a tribunal, recommending that the pharmaceutical companies be forced to license their patents on ARVs for fixed percentage royalties. Faced with the destruction of their business model if this legal theory were to spread, not just in Africa but potentially the world over, GSK and BI caved. In 2004, in exchange for TAC’s withdrawal of their Competition Commission complaint, the companies began ‘voluntarily’ licensing their ARVs to low-cost manufacturers including, in December, Cipla. In between all of this, U.S. president George W. Bush, Dubya himself, emerged as an unlikely hero, overruling objections within and outside his government to launch PEPFAR, which provides free ARVs to African countries, relying on Indian generics companies like Cipla to provide the vast majority of the supply. The ‘pirate’ had turned privateer, and the good ship Cipla had been recruited into a new armada that was finally ready to send AIDS to a watery grave.
A watery grave that was, unfortunately, already littered with the bones of the disease’s victims – and the victims of the pharma companies. See, the ARV triple-cocktail cut the US HIV/AIDS death rate in half in just a single year – 1996. Let’s say it should have taken a generous 2 years from the time of initial introduction in the US to ramp up production of the drugs and handle regulatory qualification in Africa – so we’re now in 1998. With one more year, it should have been possible to drop the death rates by that same 50% mark. Of course, none of this happened – the pharma companies refused to play ball, and the number of HIV/AIDS-related deaths *rose* between 1998 and 2003 in Africa, beginning to fall only in 2004-2005, not so coincidentally around the same time that GSK and BI finally began licensing their patents to low-cost manufacturers. On average, about 1.8 million people died of HIV/AIDS-related causes in Africa in each of the 6 years between the beginning of 1998 and the end of 2003 – if the pharma companies had done or allowed large-scale low-cost distribution of the ARVs sooner, it’s very likely that 50% of those deaths, which comes out to about 5.4 million, could have been prevented, and I have no hesitation laying those bodies at their feet. Indeed, the death toll of their intransigence might be even higher than that because of a key difference in the treatment regimens sold by the originator pharma companies and Cipla – the number of tablets that the patient needed to take per day. The pharma companies each manufactured and sold their medicines – for example, the three components of the stavudine (d4T) + lamivudine (3TC) + nevirapine (NVP) cocktail – separately as separate pills, and a single day’s dosage was usually more than one pill of each, initially as much as 7 different pills per day. Cipla, on the other hand, unencumbered by patent restrictions that kept the medicines apart in the west, manufactured a single-tablet fixed-dose combination of all three compounds, Triomune, that was found to be bioequivalent to all the medicines taken separately. Multiple studies have shown that these kinds of fixed-dose formulations, which can allow individuals to take just a single tablet a day instead of several, as much as double the percentage of the population displaying gold-standard (95%+) adherence to the treatment regimen, and consequently cut hospitalization risk (compared to multi-tablet regimens) by between 20-30%. If this translates to even a 10% additional boost to the effectiveness of the treatment regimen at preventing death due to HIV/AIDS across the overall population of patients, which would incrementally further reduce the death rate after deployment by 55% instead of 50%, the number of people who died that didn’t have to was more than 5.9 million.
This is shockingly close to the estimate of 6 million Jewish deaths during the Holocaust, one of the most horrifying legacies of the 20th century – the pharma companies could barely wait until the turn of the millennium to produce one of their very own. What were the consequences to their cold-blooded actions as an entire continent was ravaged by a pathogen, as tens of millions cried out for a precious nectar they controlled? Consider, once, again, Jean-Pierre Garnier, the head of GSK during this era. The HIV/AIDS crisis was just one of JP’s greatest hits. Here’s just a small sampling: In 2012, GSK was fined 3 billion dollars for bribing doctors to overprescribe their blockbuster antidepressant Paxil, including pushing it on young children, during JP’s tenure, even as evidence emerged that the drug made those who took it more likely to commit suicide. He is also believed to have knowingly helped cover up the fact that GSK’s diabetes drug Avandia caused heart attacks, including intimidating a professor at UNC who published research on the subject, and deliberately ignored manufacturing defects at the company’s mammoth Puerto Rico plant. What became of this shining exemplar of pharmaceutical morality, you might wonder? Remarkably, his belated limited-supply price cuts gave him a reputation as something of a humanitarian hero in France, which awarded him the Legion d'Honneur (twice!), and Great Britain, which knighted him for his trouble. He retired in 2008, telling the Guardian “je ne regrette rien” – I regret nothing – on his way out the door. JP is still alive, unlike a lot of Africans who contracted HIV in the 1990s.
Meanwhile, pharmaceutical companies continue to work off the same morally-depraved playbook to deprive the world’s poor of essential medicines. In February, the New York Times reported on a heartbreaking group of pre-teen children with cystic fibrosis right here in Hyderabad who could be saved from certain death using the miraculous new medicine Trikafta. The only problem is that its patent-owner, the Boston-based biotech company Vertex Pharmaceuticals, which sells the drug for 300k+ dollars per patient per year in the US, won’t make the drug available in India, South Africa, and most of the rest of the developing world at any price, but still won’t let other manufacturers produce or sell it in those countries either. The company has made $17 billion in revenue from Trikafta so far, and the drug was just introduced in 2019. Vertex's patents for Trikafta won't expire until 2037. Other examples abound, including J&J attempting to extend its patent protection on Bedaquiline, a last-line treatment against drug-resistant tuberculosis, by claiming that minor modifications they made and patented after their initial filing are essential to the drug’s efficacy.
Cipla, meanwhile, has been in the news lately – there is speculation that the Hamieds may sell their entire 33% promoter stake to Blackstone or another global private equity player and exit the company entirely. What alterations might this bring to the character of the company that saved so many? What happens when the old pirate captain of Bombay steps onshore for good? We can only speculate.
One thing is clear – it is essential that we in India, and indeed countries across the developing world, have indigenous next-generation pharmaceutical technologies – our own research and development, not merely the means of production, because if we do not, western pharmaceutical companies will get there first and ensure that we don’t have access for a long, long time, as we watch our brothers and sisters wither away from disease for want of medicine these companies have but refuse to provide. The solution to global vaccine and pharmaceutical inequity will not be found in Boston, Geneva, Washington, or Paris, it will be found in Hyderabad and Cape Town and Rio and Bangkok, and anyone who tells you otherwise is doomed to watch the bodies fall like dominoes once again.
Similia similibus curantur
If my writing on this subject seems too fiery, if it verges on polemic, you must excuse me – aggressive critique of global pharma is an old habit of my youth, one learned at my parents’ knees. This is not, mind you, blind adoption of a lineal belief – it is one I deliberately kept, after much consideration, among many I discarded along the way.
For my parents, both of them, are homoeopaths – disciples of what claims to be a system of medicine founded on the principle of similia similibus curantur, like cures like, that the best cure for a disease is a small amount of something that mimics its symptoms. How small an amount, you ask? Oh, so small, so miniscule, an amount so diluted in water that, technically speaking, from a chemical standpoint, there aren’t any particles of the substance left, but… eh… If you shake it very hard it um… still remains potent because of… ‘water memory’ or something?
The previous paragraphs have doubtless tipped you off, but to avoid all doubt – I don’t buy this, and I haven’t encountered any compelling evidence that homoeopathy is anything more than some combination of free-associating psychoanalysis and the unknowing exploitation of the (very real) placebo effect. Eagle-eyed readers will have noticed that, dilution below Avogadro’s limit aside, there is actually the seed of something useful here – the principle of homoeopathy is not too far from that of vaccination, a form of medicine that started on very similar lines, exposing the body to a small amount of infectious substance to prevent a real infection later on. Indeed, the progenitor of homoeopathy, the German physician Samuel Hahnemann, was a contemporary of Edward Jenner, whose seminal invention of the smallpox vaccine opened the door to modern vaccinology, and was known to have taken inspiration from his work.
Since the general distribution of Jenner’s cowpox vaccination, human smallpox never again appeared as epidemically or virulently as 40–45 years before when one city visited lost at least one half and often three-quarters of its children by death of this miserable pestilence
— Samuel Hahnemann, The Organon of the Healing Art, 6th edition, 1842
Ironically, despite Hahnemann’s admiration for vaccination, homoeopaths today seem to be – from my fairly vast anecdotal experience of them – largely against it, harbouring both deep doubts about efficacy and sinister suspicions about the intent of the researchers and companies behind them. My parents, in particular my father, number among the antivaxxers – I wasn’t vaccinated at all as a child, and I was never able to convince my father to take even a single COVID-19 vaccine dose.
For all their flaws, my parents gave me a childhood full of both love and open intellectual exploration, and one of the ways I used that freedom was to try to understand if there was a case to be made against vaccines. I had an open mind, and I already knew that there was a very real case to be made against overprescription of many kinds of very useful medicines – for example, in India in particular, antibiotic overuse and misuse has resulted in the emergence of dangerous antibiotic resistant versions of pathogens such as TB that are essentially untreatable, and claim hundreds of thousands of lives a year.
Not so with vaccines. The more I read, the more data I absorbed, the more one thing became clear: vaccination is one of the most effective public health interventions of any kind, right up there with HIV ARVs. Vaccines have cumulatively saved hundreds of millions of lives over the past few decades, most of them children who would have otherwise died very young. Consider, for example, measles. My father speaks particularly derisively of the MMR vaccine (intended to protect against measles, mumps, and rubella) which he believes causes autism – it almost certainly doesn’t, and no methodologically sound study has ever found evidence supporting this sadly popular claim – and waves off the disease as mild and inconsequential, recalling the measles parties of his youth where children were intentionally exposed at an early age.
Measles is anything but inconsequential. Prior to 1963, when the first measles vaccine was introduced, an average of 2.6 million people – mostly young children – are estimated to have died of this disease every year across the world. At the local level, that might look like 1 out of a thousand or two thousand case, but across the multibillion-strong scale of the global population, that adds up *quick*.
In India in the 1960s and 70s, while my father was growing up, measles was killing hundreds of thousands of children a year. Even as late as 1990, measles was killing nearly 150k children under the age of 5 every year – I can’t stomach that number, and the attendant grieving families who’ve had their newborns cruelly stripped away from them by something entirely preventable. Hell, even in the late 90s, after I was born, measles was killing 100k+ children per year – in a nation birthing ~10 million kids/year, that’s scarily close to 1%. My parents objected to my being vaccinated against the measles, so I could very well have been among that number.
Thankfully, as measles vaccine coverage increased from ~50% in 1990 to 90%+ in 2015, deaths from measles in India have fallen dramatically – there are not more than a few thousand per year now. That’s still too a high a number for me – we can and should bring it down to 0 – but a vast amount of death has been prevented, a huge amount of suffering averted, and millions of children have been afforded the opportunity to survive, thrive, and contribute their unique voice to this strange world of ours.
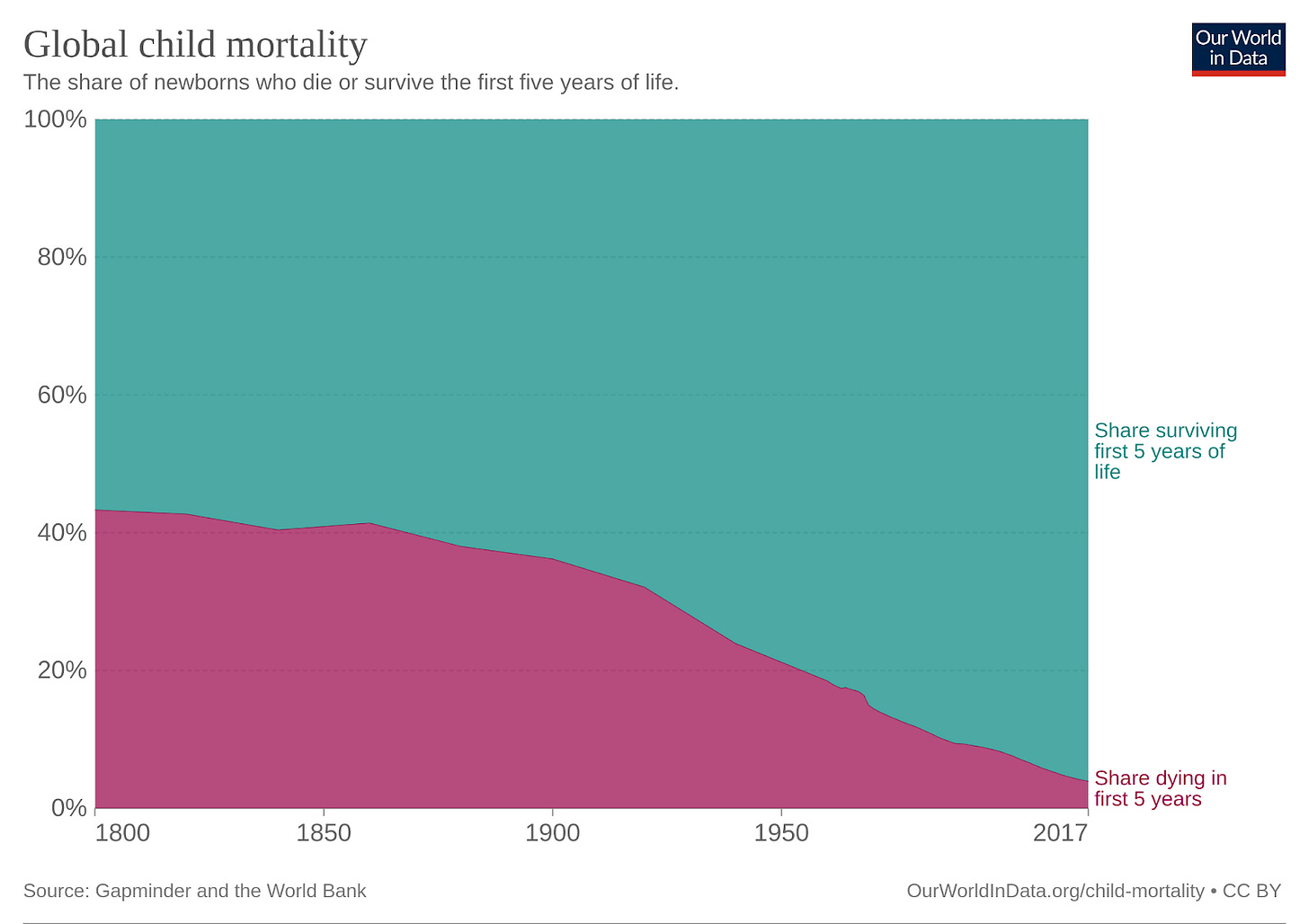
And that’s just measles – just over 200 years ago, in 1800, more than 40% of children died before the age of 5, and over 150 years later, in 1950, that number was still in excess of 20%, with most of those deaths attributable to infectious diseases in some way, shape, or form.
Today, as we’ve gotten better at preventing or blunting the impact of these diseases, we take it for granted that the vast, vast majority of children will survive their childhood, not just in the western world, but also here in India, where today ~3% of children die before the age of 5, down from ~28% in 1950. Vaccines don’t just directly prevent those deaths but, by reducing the incidence of adverse health incidents requiring hospitalization or intensive care, dramatically reduce the operational and financial burden on often already-overstretched national healthcare systems and local regional healthcare, and thus allow for effort that would have been ‘wasted’ on vaccine-preventable incidents to be reallocated to others who might now live or have significantly improved quality of life as a result. As the COVID-19 pandemic has demonstrated, even just a few additional serious cases per thousand can completely overwhelm even rich-country healthcare systems and compromise the availability and quality of care for everyone.
For most of my life, making versions of this argument across the dinner table to my family was the extent of my direct involvement in vaccinology. I spent most of my adolescence writing code and building robots while loudly forswearing any involvement in healthcare – the whole homoeopathy mess aside, the hours seemed utterly ludicrous – and then, wanting absolutely nothing to do with competitive entrance exams, shipped myself off to America at 17 to start an undergraduate degree in Computer Science at Yale, followed by a PhD program in the same subject at Cornell, focusing once again on robotics. That state of affairs remained entirely undisturbed until 2020, the first year of the pandemic. What changed? Well, my return to the vaccine battleground – this time as active combatant rather than as non-partisan observer turned booster (no pun intended) – owes a lot to one graph in particular. Here it is below – can you spot the strange pattern that caught my eye?

More on this, and the other two meetings we began with, in Part II, which will be released next week. If you want to hear about it as soon as it comes out, subscribe below.
And, of course, if you’re interested in working at PopVax, take a look at our open positions at jobs.popvax.com. If you believe you could contribute to the company in some way that’s not captured in those job descriptions, you’re welcome to email a cover letter and resume to jobs@popvax.com.
Please feel free to email questions, comments, or feedback about this post to newsletter@popvax.com.
You can also follow us on X/Twitter at @PopVaxIndia.